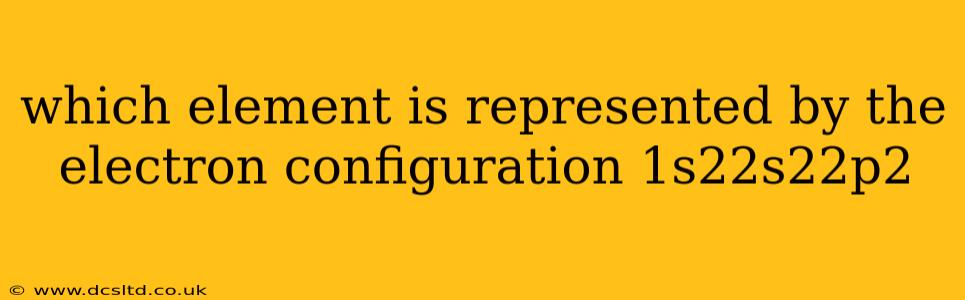
Which Element is Represented by the Electron Configuration 1s²2s²2p²?
The electron configuration 1s²2s²2p² represents the element Carbon (C). Let's break down why:
Electron configurations describe the arrangement of electrons in an atom's electron shells and subshells. Each number and letter represents a specific orbital:
-
1s²: This indicates two electrons in the first energy level (n=1), specifically in the s subshell. The s subshell can hold a maximum of two electrons.
-
2s²: This shows two electrons in the second energy level (n=2), in the s subshell. Again, the s subshell holds a maximum of two electrons.
-
2p²: This signifies two electrons in the second energy level (n=2), within the p subshell. The p subshell can hold up to six electrons, but in this case, only two are present.
Adding up the electrons (2 + 2 + 2 = 6), we find that the atom has six electrons. Since atoms are electrically neutral, the number of electrons equals the number of protons, which defines the atomic number. Carbon has an atomic number of 6, making it the element represented by this electron configuration.
What are the properties of Carbon based on this electron configuration?
Carbon's electron configuration explains several of its key properties:
-
Tetravalency: Carbon has four electrons in its outermost shell (the valence shell). This means it can form four covalent bonds with other atoms, leading to a vast array of compounds and its importance in organic chemistry.
-
Bonding versatility: The ability to form single, double, and triple bonds allows carbon to create diverse structures, including chains, rings, and branched molecules.
-
Allotropes: Carbon's ability to form different structural arrangements (allotropes) gives rise to materials with vastly different properties. Diamond, graphite, and fullerenes are prime examples of carbon allotropes, highlighting the unique bonding possibilities of this element.
How do I determine the element from an electron configuration?
To identify an element from its electron configuration, simply add up the total number of electrons. This number represents the element's atomic number, which you can then use to look up the element on the periodic table.
What are some other elements with similar electron configurations?
While the exact configuration 1s²2s²2p² is unique to Carbon, other elements in the same group (Group 14 or IVA) will have similar valence electron configurations (outermost shell electrons), resulting in comparable chemical behavior, though their properties will differ significantly due to the increased number of inner electrons and energy levels. For instance, Silicon (Si) has an electron configuration that ends in 3s²3p², showing a similar valence electron arrangement.
What are some common applications of Carbon?
Carbon's diverse properties lead to wide-ranging applications. It’s a crucial component of:
- Organic chemistry: The backbone of all organic molecules.
- Materials science: Used in diamonds (abrasives, jewelry), graphite (lubricants, pencils), and carbon fiber (strong, lightweight composites).
- Energy: Used in fuels and as a component in batteries.
- Medicine: Used in various medical imaging and treatment applications.
Understanding the electron configuration of an element is key to predicting its chemical behavior and properties. Carbon's simple yet powerful configuration is the foundation for a vast world of chemistry and material science.