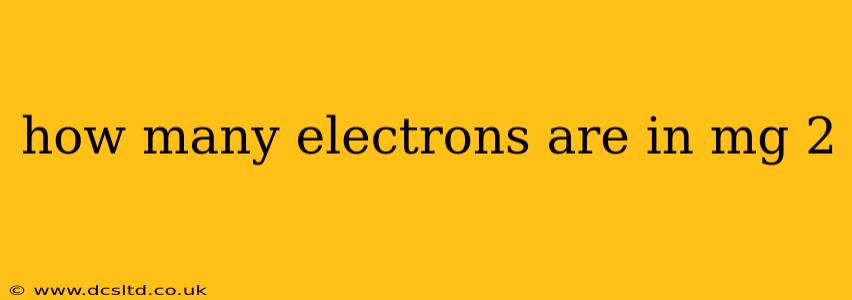
How Many Electrons Are in Mg²⁺?
Magnesium (Mg) is an element with an atomic number of 12, meaning a neutral magnesium atom has 12 protons and 12 electrons. The superscript "2+" in Mg²⁺ indicates that this is a magnesium ion that has lost two electrons. Therefore, a Mg²⁺ ion has 10 electrons.
Let's break this down further:
What Happens When Magnesium Forms an Ion?
Magnesium is a metal and readily loses electrons to achieve a stable electron configuration. It's in group 2 of the periodic table, meaning it has two valence electrons in its outermost shell. These are the electrons most easily lost. To gain stability, magnesium readily gives up these two valence electrons to become a 2+ cation (positively charged ion).
Calculating Electrons in Ions
To determine the number of electrons in any ion:
- Find the atomic number: This tells you the number of protons and electrons in a neutral atom. For magnesium, the atomic number is 12.
- Consider the charge: The charge on the ion indicates the number of electrons lost (positive charge) or gained (negative charge). Mg²⁺ has a charge of +2, meaning it has lost two electrons.
- Subtract (for positive ions) or add (for negative ions): Subtract the charge number from the atomic number for positive ions. For Mg²⁺, 12 (atomic number) - 2 (charge) = 10 electrons.
Why is this important?
Understanding the number of electrons in ions is crucial for comprehending:
- Chemical bonding: Ions with opposite charges attract each other, forming ionic bonds which are fundamental to the structure of many compounds.
- Chemical reactions: The number of electrons determines how an ion will interact with other atoms and molecules.
- Properties of matter: The electron configuration dictates many of the physical and chemical properties of substances.
Frequently Asked Questions (FAQ)
What is the electron configuration of Mg²⁺?
The electron configuration of neutral magnesium is 1s²2s²2p⁶3s². After losing two electrons, the Mg²⁺ ion has an electron configuration of 1s²2s²2p⁶, which is the same as neon (Ne), a noble gas. This stable configuration is why magnesium readily forms this ion.
How many protons are in Mg²⁺?
The number of protons remains unchanged when an atom becomes an ion. Therefore, Mg²⁺ still has 12 protons.
What are some common compounds containing Mg²⁺?
Magnesium ions are found in many compounds, including magnesium oxide (MgO), magnesium chloride (MgCl₂), and magnesium sulfate (MgSO₄).
In summary, a Mg²⁺ ion has 10 electrons, a consequence of losing two electrons from a neutral magnesium atom to achieve a stable electron configuration. Understanding this is fundamental to comprehending the behavior of magnesium in chemical reactions and its role in various compounds.